Dosing
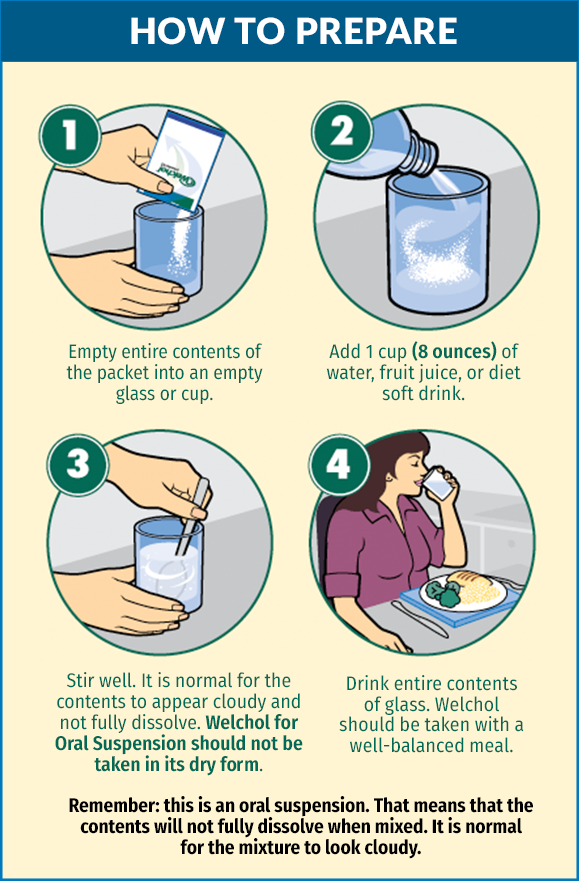
Welchol for Oral Suspension offers mixing options for your patients


*Phenylketonurics: Welchol for Oral Suspension contains 27 mg phenylalanine per 3.75 g dose. Phenylalanine can be harmful to patients with
phenylketonuria (PKU).
The Welchol for Oral Suspension dosing option gives your patients1:
- Customization with a variety of mixing options (water, fruit juice, or diet soft drinks)
- Dosing flexibility to be taken with any meal of the day (preferably the same meal daily)
- Taste—Welchol for Oral Suspension is sugar-free and citrus-flavored
Welchol for Oral Suspension should not be taken in its dry form. Due to tablet size, Welchol for Oral Suspension is recommended for, but not limited to, use in the pediatric population as well as in any patient who has difficulty swallowing tablets.

Dosage in type 2 diabetes
- The recommended dose of Welchol for Oral Suspension is 1 packet once a day
- Welchol for Oral Suspension should be taken with a meal
Dosage in primary hyperlipidemia
- The recommended dose of Welchol for Oral Suspension is 1 packet once a day
- Welchol for Oral Suspension should be taken with a meal
- Welchol for Oral Suspension can be dosed at the same time as an HMG-CoA reductase inhibitor (statin) or the two drugs can be dosed apart
- After initiation of Welchol, lipid levels should be analyzed within 4 to 6 weeks
- Welchol for Oral Suspension is recommended for, but not limited to, the pediatric population and for any patient who has difficulty swallowing tablets. Dose adjustments are not required when Welchol is administered to children 10-17 years of age
- Welchol has not been studied in children younger than 10 years of age or in pre-menarchal girls
Dosage form and strength
- Each 3.75 g oral suspension packet contains a single dose
Contraindications:
Welchol is contraindicated in patients with:
- Serum TG concentrations >500 mg/dL
- A history of hypertriglyceridemia-induced pancreatitis
- A history of bowel obstruction
Please see Important Safety Information about Welchol below.
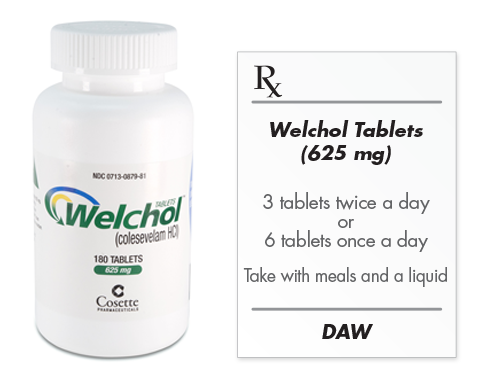
Welchol Tablets
A1C and LDL-C efficacy without systemic absorption for your adult patients with T2DM and primary hyperlipidemia

The Welchol Tablet dosing option gives your patients1:
- Dosing flexibility to be taken with any meal of the day (preferably the same meal daily)
- Adjustability with a once- or twice-daily* dosing regimen
*Twice-daily dosing only applies to Welchol Tablets.
Dosage in primary hyperlipidemia and T2DM
- The recommended dose of Welchol Tablets in adults, whether used as monotherapy or in combination with an HMG-CoA reductase inhibitor (statin), is 6 tablets once daily or 3 tablets twice daily
- Welchol Tablets should be taken with a meal and liquid
- Welchol for Oral Suspension is available as an alternative—3.75 g dose that mixes with 4-8 ounces of water, fruit juice, or diet soft drinks and should be taken with a meal
- Welchol can be dosed at the same time as an HMG-CoA reductase inhibitor (statin) or the two drugs can be dosed apart
- After initiation of Welchol, lipid levels should be analyzed within 4 to 6 weeks
Dosage form and strength
Due to tablet size, Welchol for Oral Suspension is recommended for, but not limited to, use in the pediatric population as well as in any patient who has difficulty swallowing tablets.
- 625 mg tablets are off-white, oval, film-coated, and imprinted with "Sankyo" and "C01" on one side
Contraindications
Welchol is contraindicated in patients with:
- A history of bowel obstruction
- Serum TG concentrations >500 mg/dL
- A history of hypertriglyceridemia-induced pancreatitis
Please see Important Safety Information about Welchol below.
Welchol Savings for Your Patients
Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW

Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW
Welchol is indicated as an adjunct to diet and exercise to:
- reduce elevated low-density lipoprotein cholesterol (LDL-C) in patients with primary hyperlipidemia
- reduce LDL-C levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH)
- improve glycemic control in adults with type 2 diabetes mellitus
- Welchol should not be used for the treatment of type 1 diabetes or for the treatment of diabetic ketoacidosis
- The effect of Welchol on cardiovascular morbidity and mortality has not been determined.
- Welchol has not been studied in type 2 diabetes in combination with a dipeptidyl peptidase-4 inhibitor
- Welchol has not been studied in Fredrickson Type I, III, IV, and V dyslipidemias
- Welchol has not been studied in children younger than 10 years of age or in premenarchal girls
To the Patient: You must present this card to the pharmacist along with your WELCHOL® (colesevelam HCl) prescription to participate in the program. For patients with commercial insurance, savings per prescription of WELCHOL will apply after the following out‐of‐pocket expenses are met: $10 per prescription for a 30‐day supply of WELCHOL or $0 per prescription for a 90‐day supply of WELCHOL. Offer may not be combined with any other program offer or discount for WELCHOL. Savings for WELCHOL are subject to a maximum benefit of $150 per 30‐day prescription or $450 per 90‐day prescription. If you have questions regarding your eligibility or benefits, or wish to discontinue participation, call (877) 264‐2440
(8 AM – 8 PM ET, Monday‐Friday). When you use this card, you are certifying that you understand the program rules, regulations, and terms and conditions. You are not eligible if you are enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS; or where taxed, restricted, or prohibited by law; or if you do not otherwise comply with the terms of this card. Further, you agree to discontinue using the card if you enroll in any state or federal health care program during the program period. Offer valid in US and Puerto Rico only.
To the Pharmacist: When you use this card, you are certifying that the patient is not enrolled in any federal, state, or other governmental programs for this prescription.
- Submit transaction to McKesson Corporation, using BIN #610524.
- If primary coverage exists, input card information as secondary coverage and transmit using the COB segment of NCPDP transaction. Applicable discounts will be displayed in the transaction response.
- Acceptance of this card is subject to LoyaltyScript® program Terms and Conditions posted at www.mckesson.com/mprstnc.
- Patient not eligible if enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS, or where taxed, restricted, or prohibited by law. Offer valid in US and Puerto Rico only.
- The LoyaltyScript® card is not valid for use with any other prescription drug discount or cash cards for WELCHOL. Claims submitted utilizing the program are subject to audit or validation.
- LoyaltyScript® is not an insurance card.
Cosette Pharmaceuticals, Inc., reserves the right to rescind, revoke, or amend this program, at any time, without notice.
Trademarks not owned by Cosette Pharmaceuticals, Inc., are property of their respective owners.
REFERENCE:
1. Welchol (colesevelam HCI). Prescribing Information. Cosette Pharmaceuticals, Inc., South Plainfield, NJ; 2019.
© 2022 Cosette Pharmaceuticals, Inc. CP-US-WC-004 06/22

