Safety Profile
Safety Profile
Welchol (colesevelam HCl) is contraindicated in patients with:- Serum TG concentrations >500 mg/dL
- A history of hypertriglyceridemia-induced pancreatitis
- A history of bowel obstruction
Welchol may increase triglyceride levels, especially in patients on a sulfonylurea- or insulin-based therapy. Periodic monitoring of lipid parameters including TG and non-HDL-C levels is recommended. The long-term effect of hypertriglyceridemia on the risk of coronary artery disease is uncertain. Hypertriglyceridemia can cause acute pancreatitis. Instruct patients to discontinue Welchol and seek prompt medical attention if the symptoms of acute pancreatitis occur. Caution should be exercised when treating patients with TG levels > 300 mg/dL.
Welchol may decrease the absorption of fat-soluble vitamins A, D, E, and K. Patients on oral vitamin supplements should take their vitamins at least 4 hours prior to Welchol. Caution should be exercised when treating patients with a susceptibility to vitamin K deficiencies (eg, patients on warfarin, patients with malabsorption syndromes) or fat-soluble vitamin deficiencies.
Due to tablet size, Welchol for Oral Suspension is recommended for, but not limited to, any patient who has difficulty swallowing tablets.
To avoid esophageal distress, Welchol for Oral Suspension should not be taken in its dry form.
Please see Important Safety Information about Welchol below.
Most common adverse events1
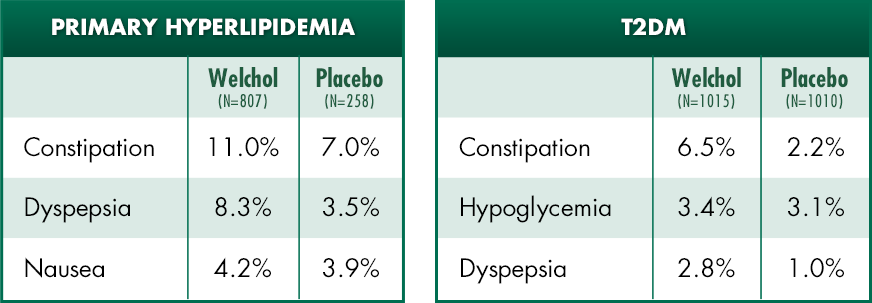
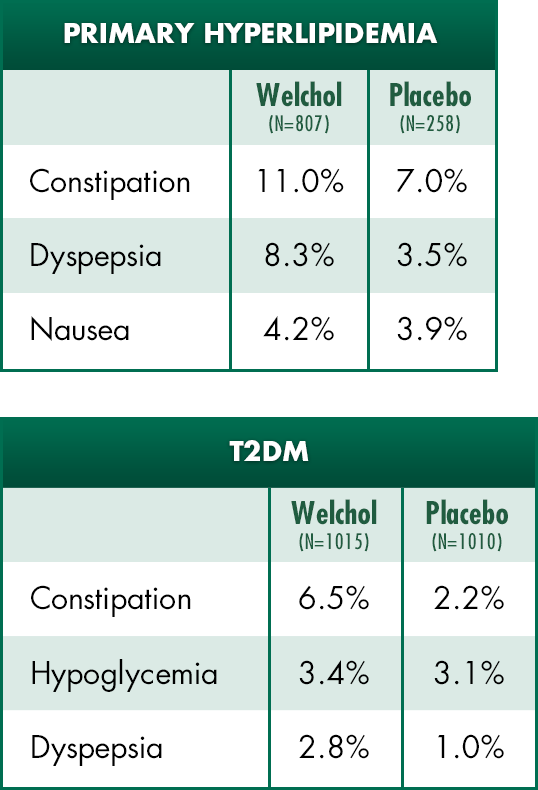
In type 2 diabetes clinical studies:
- The incidence of hypoglycemia was similar between Welchol and placebo groups (3.4% vs 3.1%), respectively
- Welchol was not associated with weight gain
How Welchol is not systemically absorbed1
Welchol binds to bile acids in the intestine without being metabolized by the liver and kidneys. Welchol does not enter the bloodstream, so you can provide add-on efficacy without systemic absorption.1

Cases of bowel obstruction have occurred. Welchol is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction.
No dosage adjustment is required in patients with hepatic impairment or mild to moderate renal impairment. Welchol has not been studied in severe renal impairment.
Welchol interacts with some drugs. Drugs with a known interaction with colesevelam (cyclosporine, glimepiride, glipizide, glyburide, levothyroxine, olmesartan medoxomil, oral contraceptives [ethinyl estradiol, norethindrone], and metformin extended release [ER]) should be administered at least 4 hours prior to Welchol. Drugs that have not been tested for interaction with colesevelam, especially those with a narrow therapeutic index, should also be administered at least 4 hours prior to Welchol. Alternatively, the physician should monitor drug levels of the co-administered drug.
Welchol Savings for Your Patients
Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW

Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW
Welchol is indicated as an adjunct to diet and exercise to:
- reduce elevated low-density lipoprotein cholesterol (LDL-C) in patients with primary hyperlipidemia
- reduce LDL-C levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH)
- improve glycemic control in adults with type 2 diabetes mellitus
- Welchol should not be used for the treatment of type 1 diabetes or for the treatment of diabetic ketoacidosis
- The effect of Welchol on cardiovascular morbidity and mortality has not been determined.
- Welchol has not been studied in type 2 diabetes in combination with a dipeptidyl peptidase-4 inhibitor
- Welchol has not been studied in Fredrickson Type I, III, IV, and V dyslipidemias
- Welchol has not been studied in children younger than 10 years of age or in premenarchal girls
To the Patient: You must present this card to the pharmacist along with your WELCHOL® (colesevelam HCl) prescription to participate in the program. For patients with commercial insurance, savings per prescription of WELCHOL will apply after the following out‐of‐pocket expenses are met: $10 per prescription for a 30‐day supply of WELCHOL or $0 per prescription for a 90‐day supply of WELCHOL. Offer may not be combined with any other program offer or discount for WELCHOL. Savings for WELCHOL are subject to a maximum benefit of $150 per 30‐day prescription or $450 per 90‐day prescription. If you have questions regarding your eligibility or benefits, or wish to discontinue participation, call (877) 264‐2440
(8 AM – 8 PM ET, Monday‐Friday). When you use this card, you are certifying that you understand the program rules, regulations, and terms and conditions. You are not eligible if you are enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS; or where taxed, restricted, or prohibited by law; or if you do not otherwise comply with the terms of this card. Further, you agree to discontinue using the card if you enroll in any state or federal health care program during the program period. Offer valid in US and Puerto Rico only.
To the Pharmacist: When you use this card, you are certifying that the patient is not enrolled in any federal, state, or other governmental programs for this prescription.
- Submit transaction to McKesson Corporation, using BIN #610524.
- If primary coverage exists, input card information as secondary coverage and transmit using the COB segment of NCPDP transaction. Applicable discounts will be displayed in the transaction response.
- Acceptance of this card is subject to LoyaltyScript® program Terms and Conditions posted at www.mckesson.com/mprstnc.
- Patient not eligible if enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS, or where taxed, restricted, or prohibited by law. Offer valid in US and Puerto Rico only.
- The LoyaltyScript® card is not valid for use with any other prescription drug discount or cash cards for WELCHOL. Claims submitted utilizing the program are subject to audit or validation.
- LoyaltyScript® is not an insurance card.
Cosette Pharmaceuticals, Inc., reserves the right to rescind, revoke, or amend this program, at any time, without notice.
Trademarks not owned by Cosette Pharmaceuticals, Inc., are property of their respective owners.
REFERENCE:
1. Welchol (colesevelam HCI). Prescribing Information. Cosette Pharmaceuticals, Inc., South Plainfield, NJ; 2019.
© 2022 Cosette Pharmaceuticals, Inc. CP-US-WC-004 06/22

