Welchol Clinical Studies
LDL-C and A1C reductions
In the appropriate patient, as an adjunct to diet and exercise, Welchol is the only bile acid sequestrant FDA-approved to provide both LDL-C and A1C reductions in adults with primary hyperlipidemia and T2DM. See how Welchol compares to atorvastatin, simvastatin, and metformin.
WELCHOL + ATORVASTATIN 10 mg:
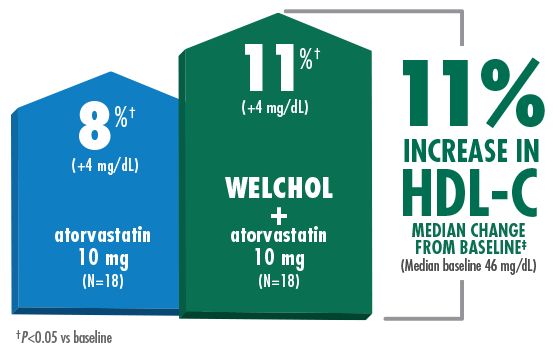
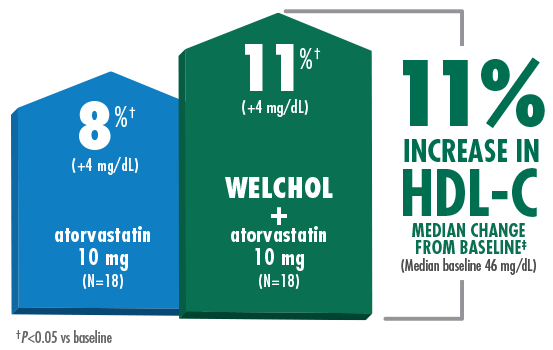
Lowered LDL-C and raised HDL-C without statin titration
Adding Welchol to atorvastatin 10 mg significantly lowered LDL-C, comparable to atorvastatin 80 mg1,2
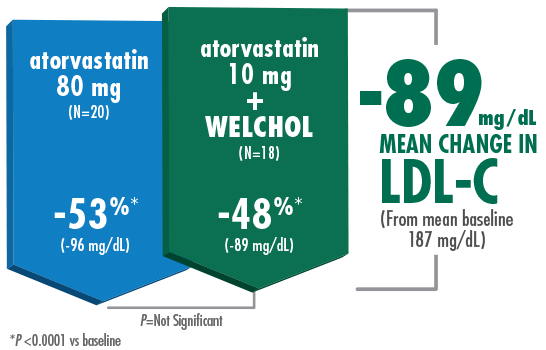
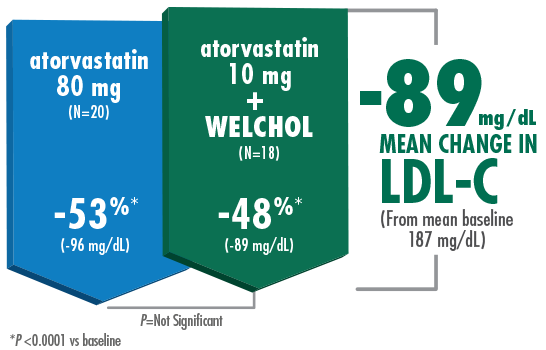
- The most common adverse events were flatulence (26%) and constipation (21%) in the Welchol + atorvastatin 10 mg group and
infection (24%) in the Welchol 3.8 g alone group2 - T2DM was not an inclusion criterion for this study
- Reduction in HDL-C was a secondary endpoint in this study and Welchol is not indicated to raise HDL-C
‡Welchol (N=16) vs placebo (N=19).
Hunninghake D, et al. Atherosclerosis. 2001. Please see study design B.
As an add-on,
Welchol + atorvastatin 10 mg lowered APO B by 38%1,2
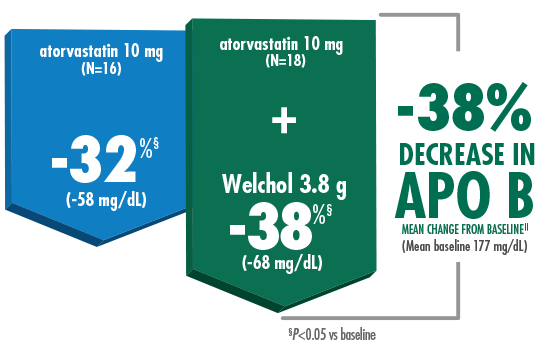
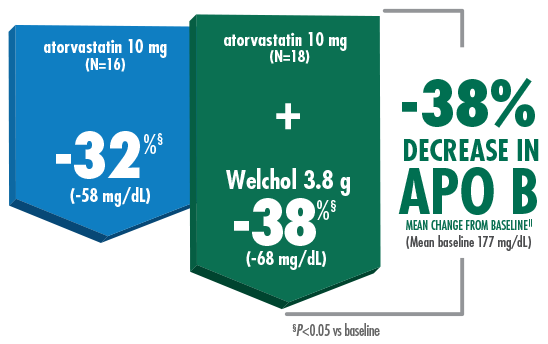
Welchol is not indicated to lower APO B. APO B was a secondary endpoint in this study.
- T2DM was not an inclusion criterion for this study
- The most common reported adverse events were flatulence (26%) and constipation (21%) in the Welchol + atorvastatin 10 mg group and infection (24%) in the Welchol 3.8 g alone group2
ǁWelchol (N=16) vs placebo (N=19).
At baseline, 68% of patients had LDL cholesterol levels <190 mg/dl, and 32% of patients had LDL cholesterol levels >190 mg/dl.2
Hunninghake D, et al. Atherosclerosis. 2001. Please see study design B.
Study Design B (Hunninghake D, Atherosclerosis. 2001) Results from a randomized, double-blind, placebo-controlled, 4-week study of 94 randomized patients with moderate hypercholesterolemia¶ (LDL-C ≥160 mg/dL, TG ≤300 mg/dL) and LDL-C levels ranging from 156-236 mg/dL. Patients were assigned to 1 of 5 treatment groups: placebo, Welchol 3.8 g/day, atorvastatin 10 mg/day, atorvastatin 80 mg/day, or atorvastatin 10 mg/day + Welchol 3.8 g/day.1,2
¶Hypercholesterolemia is now referred to as primary hyperlipidemia.
Please see Important Safety Information about Welchol below.
Welchol & Simvastatin Clinical Study
42% mean reduction in LDL-C and 10% median increase in HDL-C when Welchol 3.8 g was added to simvastatin 10 mg1,3
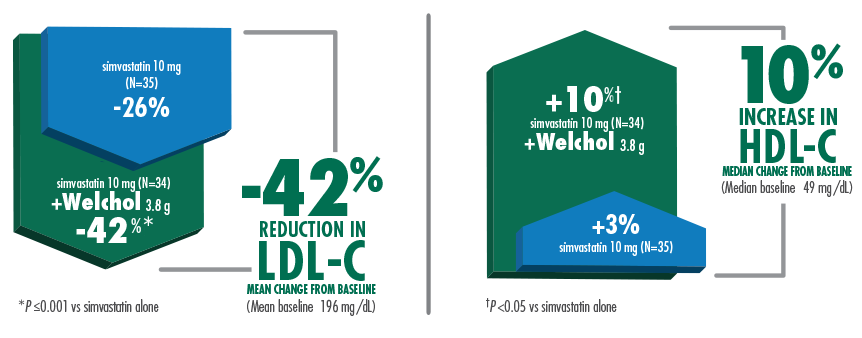
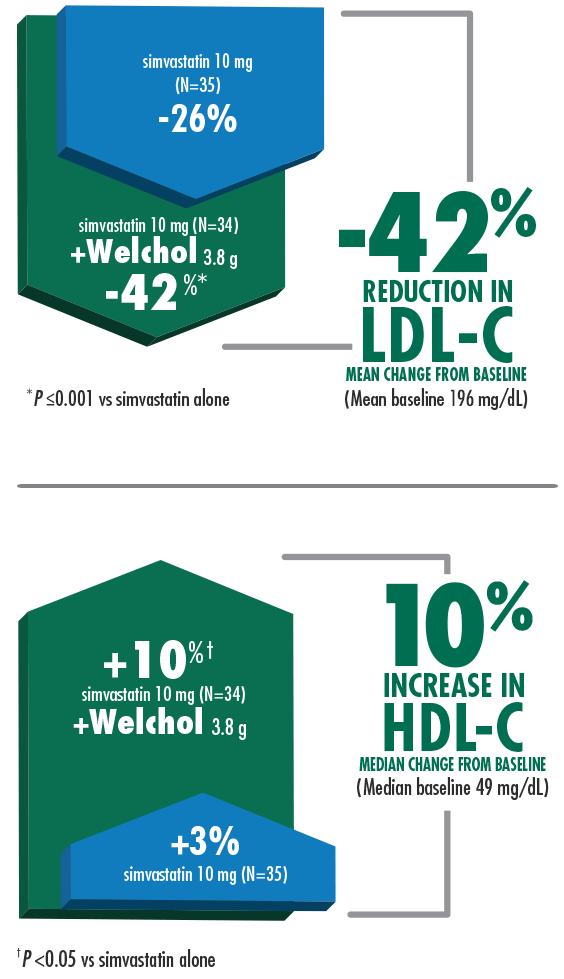
Welchol is not indicated to raise HDL-C. HDL-C was a secondary endpoint in this study.
T2DM was not an inclusion criteria for these studies.
The most common adverse events (≥10%) were flatulence and constipation.
Please see Important Safety Information about Welchol below.


Treatment groups had similar baseline characteristics, except for serum triglyceride levels (P <0.05). Among study patients, 62% (n=155) had fewer than two risk factors for coronary heart disease, and 26% (n=66) had two or more risk factors, whereas 12% (n=30) had preexisting coronary heart disease.3
The effect of Welchol on cardiovascular morbidity and mortality has not been determined.
Knapp HH, et al. Am J Med. 2001. Please see study design A.
Study Design A (Knapp HH, Am J Med. 2001)
Results from a 6-week, multicenter, randomized, double-blind, placebo-controlled study of 258 randomized patients with moderate hypercholesterolemia* (LDL-C ≥160 mg/dL; TG ≤300 mg/dL) who were not on lipid-lowering medication. Patients were randomized to 1 of the following treatment regimens: placebo, Welchol 3.8 g/day, simvastatin 10 mg/day, Welchol 3.8 g/day + simvastatin 10 mg/day, Welchol 2.3 g/day, simvastatin 20 mg/day, or Welchol 2.3 g/day + simvastatin 20 mg/day.1,3
*Hypercholesterolemia is now referred to as primary hyperlipidemia.
Welchol + Metformin
In a type 2 diabetes pivotal trial,
Welchol provided significant A1C and LDL-C mean treatment reductions when added to metformin and metformin combination therapies1,5
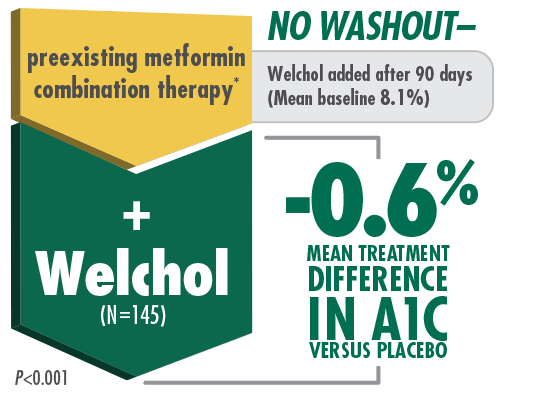



* Welchol has not been studied in combination with all antidiabetic agents.
† Reductions in A1C represent the mean treatment difference in Welchol (n=69) vs placebo (n=76). Reductions in LDL-C represent the mean treatment difference in Welchol (n=59) vs placebo (n=65).
Bays HE, et al. Arch Intern Med. 2008. Please see study design A.
Welchol + metformin monotherapy resulted in a mean treatment difference in A1C of -0.5%, N=155 (Welchol n=79, placebo n=76) (P=0.002)1
- The most common treatment-emergent adverse event in ≥5% of patients was constipation5
- Welchol was not associated with weight gain, and incidence of hypoglycemia was comparable to placebo5
Welchol has been shown to increase levels of metformin when coadministered with metformin extended release (ER).1
Welchol can increase triglycerides so lipids, including TGs and non-HDL-C, should be monitored. Welchol can increase serum TG concentrations particularly when used in combination with pioglitazone, sulfonylureas, or insulin. Caution should be exercised when treating patients with TG levels >300 mg/dL.
Because of its constipating effect, Welchol should not be used in patients at risk of bowel obstruction.
Adding Welchol may help patients reach their A1C and LDL-C goals
In a post-hoc analysis of the Bays pivotal study, Welchol + metformin combination therapy (N=69) vs metformin combination alone (N=76), adding Welchol helped some patients achieve the ADA‡ and NCEP§ goals for A1C and LDL-C (n=17, n=4, respectively).1,6,7
In the same post-hoc analysis, 20% of patients on Welchol + metformin monotherapy (N=79; n=16) vs 7% of patients on metformin monotherapy (N=76; n=5) achieved a reduction in A1C to <7%. Additionally, 26% of patients on Welchol + metformin monotherapy (N=66; n=17) vs 20% of patients on metformin monotherapy (N=61; n=12) achieved an LDL-C <70 mg/dL.
Please see Important Safety Information about Welchol below.
‡ADA=American Diabetes Association.
§NCEP=National Cholesterol Education Program.
Baseline demographic characteristics for the total population revealed no significant differences between the colesevelam and placebo groups at randomization. When other oral anti-DM drugs were used in combination with background metformin, most were sulfonylureas and thiazolidinediones.5
Study Design A (Bays HE, Arch Intern Med. 2008)
Results from a double-blind, 26-week, placebo-controlled pivotal study of 316 randomized patients with inadequate glycemic control (baseline A1C ≥7.5% and ≤9.5%). Patients were enrolled and maintained on their preexisting metformin-based therapy. Either Welchol or placebo was added to metformin alone or metformin in combination with other anti-diabetic therapies for 26 weeks. The primary efficacy endpoint was mean change in A1C from baseline; secondary endpoints included mean change in LDL-C from baseline.5
Study Design A1 (Post-hoc analysis of the Bays pivotal study) This post-hoc analysis evaluated the percentages of patients achieving A1C and LDL-C goals. Of the total number of patients (N=69) in the Welchol + metformin combination therapy group, 17 patients (25%) achieved a reduction in A1C to <7% compared with 4 patients (5%) in the metformin combination therapy group (N=76). Additionally, 22 patients (37%) in the Welchol + metformin combination therapy group (N=59) and 8 patients (12%) in the metformin combination therapy group (N=65) achieved an LDL-C <70 mg/dL.6
Welchol Savings for Your Patients
Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW

Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply. Restrictions apply based on eligibility. See Terms & Conditions below.
PRINT SAVINGSCARD NOW
Welchol is indicated as an adjunct to diet and exercise to:
- reduce elevated low-density lipoprotein cholesterol (LDL-C) in patients with primary hyperlipidemia
- reduce LDL-C levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH)
- improve glycemic control in adults with type 2 diabetes mellitus
- Welchol should not be used for the treatment of type 1 diabetes or for the treatment of diabetic ketoacidosis
- The effect of Welchol on cardiovascular morbidity and mortality has not been determined.
- Welchol has not been studied in type 2 diabetes in combination with a dipeptidyl peptidase-4 inhibitor
- Welchol has not been studied in Fredrickson Type I, III, IV, and V dyslipidemias
- Welchol has not been studied in children younger than 10 years of age or in premenarchal girls
To the Patient: You must present this card to the pharmacist along with your WELCHOL® (colesevelam HCl) prescription to participate in the program. For patients with commercial insurance, savings per prescription of WELCHOL will apply after the following out‐of‐pocket expenses are met: $10 per prescription for a 30‐day supply of WELCHOL or $0 per prescription for a 90‐day supply of WELCHOL. Offer may not be combined with any other program offer or discount for WELCHOL. Savings for WELCHOL are subject to a maximum benefit of $150 per 30‐day prescription or $450 per 90‐day prescription. If you have questions regarding your eligibility or benefits, or wish to discontinue participation, call (877) 264‐2440
(8 AM – 8 PM ET, Monday‐Friday). When you use this card, you are certifying that you understand the program rules, regulations, and terms and conditions. You are not eligible if you are enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS; or where taxed, restricted, or prohibited by law; or if you do not otherwise comply with the terms of this card. Further, you agree to discontinue using the card if you enroll in any state or federal health care program during the program period. Offer valid in US and Puerto Rico only.
To the Pharmacist: When you use this card, you are certifying that the patient is not enrolled in any federal, state, or other governmental programs for this prescription.
- Submit transaction to McKesson Corporation, using BIN #610524.
- If primary coverage exists, input card information as secondary coverage and transmit using the COB segment of NCPDP transaction. Applicable discounts will be displayed in the transaction response.
- Acceptance of this card is subject to LoyaltyScript® program Terms and Conditions posted at www.mckesson.com/mprstnc.
- Patient not eligible if enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS, or where taxed, restricted, or prohibited by law. Offer valid in US and Puerto Rico only.
- The LoyaltyScript® card is not valid for use with any other prescription drug discount or cash cards for WELCHOL. Claims submitted utilizing the program are subject to audit or validation.
- LoyaltyScript® is not an insurance card.
Cosette Pharmaceuticals, Inc., reserves the right to rescind, revoke, or amend this program, at any time, without notice.
Trademarks not owned by Cosette Pharmaceuticals, Inc., are property of their respective owners.
REFERENCES:
1. Welchol (colesevelam HCI). Prescribing Information. Cosette Pharmaceuticals, Inc., South Plainfield, NJ; 2019.
2. Hunninghake D, Insull W Jr, Toth P, et al. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis. 2001;158:407-416.
3. Knapp HH, Schrott H, Ma P, et al. Efficacy and safety of combination simvastatin and colesevelam in patients with primary hypercholesterolemia. Am J Med. 2001;110(5):352-360.
4. US Food and Drug Administration. FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury.
http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm.Published June 8, 2011. Accessed June 18, 2019.
5. Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168(18):1975-1983.
6. Data on file. Cosette Pharmaceuticals, Inc., South Plainfield, NJ.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11-S66.
© 2022 Cosette Pharmaceuticals, Inc. CP-US-WC-004 06/22

